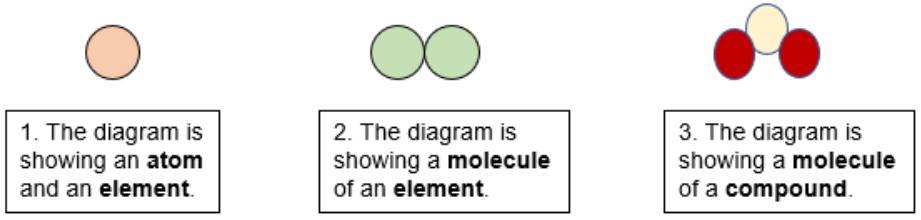
How To Determine Element Or Compound
This helps determine the oxidation state of any one element in a given molecule or ion assuming that we know the common oxidation states of all of the other elements. Use the periodic table to determine the atomic mass of each element in the molecule.
Chemistry Elements Compounds And Mixtures
Classified as a transition metal Nickel is a solid at room temperature.
How to determine element or compound. First determine how the compound splits apart into ions when it dissolves. The oxidation number of a monatomic ion equals the charge of the ion. The oxidation number of O in compounds is usually -2 but it is -1 in peroxides.
The oxidation number of H is 1 but it is -1 in when combined with less electronegative elements. This step-by-step tutorial shows how to calculate the empirical and molecular formulas for a compound. Determine the atomic weight of element M and identify it by name.
The oxidation number of a free element is always 0. The chloride of an unknown metal is believed to have the formula MCl 3A 1603 g sample of the compound is found to contain 003606 mol of Cl. We reviewed the top compound bows for the money youth beginner hunters and hunting.
You only need to know or look up the charge on 1 ion since you know the total compound will. Multiply each elements atomic mass by the number of atoms of that element in the molecule. This article considers the origin of the elements and their abundances throughout the universeThe geochemical distribution of these elementary substances in the Earths crust and interior.
The shorthand notation for a compound describes the number of atoms of each element which is indicated by a subscript written after the symbol for the element. If we burn 100 g of this compound to produce 150 g of CO 2 and 041 g of H 2 O what is the empirical formula of the compound. We also explained the choosing factors like purpose speed achieving accuracy weight let-off draw length and more.
The molecular formula is the representation of the actual whole number ratio between the elements of the compound. Use each elements molar mass to convert the grams of each element to moles. Nitride any of a class of chemical compounds in which nitrogen is combined with an element of similar or lower electronegativity such as boron silicon and most metals.
For example a molecule of PbI 2 splits into the ions Pb 2 I- and a second I-. Next write an equation with the K sp on one side and the constituent ions on the other. Steps to determine empirical formula.
For example in a sulfite ion SO 3 2- the total charge of the ion is 2- and each oxygen is assumed to be in its usual oxidation state of -2. This video demonstrates the steps that are to be taken for the experimental determination of the empirical formula for magnesium oxide. This number is represented by the subscript next to the element symbol in the molecular formula.
Nickel is a chemical element with symbol Ni and atomic number 28. Chemical element also called element any substance that cannot be decomposed into simpler substances by ordinary chemical processesElements are the fundamental materials of which all matter is composed. In chemistry an element is a pure substance consisting only of atoms that all have the same numbers of protons in their nucleiUnlike chemical compounds chemical elements cannot be broken down into simpler substances by any chemical reactionThe number of protons in the nucleus is the defining property of an element and is referred to as its atomic number represented by the symbol Z.
Determine the molecular formula of the molecule. Assume a 100. Nitrides contain the nitride ion N3 and similar to carbides nitrides can be classified into three general categories.
The oxidation number of a Group 1 element in a compound is 1. To determine the empirical formula of this compound we must first calculate the masses of C H and O. The empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound.
By convention no subscript is written when a molecule contains only one atom of an element. Textg sample of the compound so that the given percentages can be directly converted into grams. Want to know the best compound bows of 2021.
Imagine that we have an organic compound that contains C H and O. Thus water is H 2 O and carbon dioxide is CO 2. A given element is said to match a complex selector when there exists a list of elements each matching a corresponding compound selector in the complex selector with each pair of elements consecutive in the list matching the combinator between their corresponding compound selectors and with the last element being the given element.
Understanding Atoms Elements And Compounds Lesson And Worksheets

Compound Vs Mixture Difference And Comparison Diffen Compounds Science Compounds And Mixtures Chemistry Classroom

Synthesis Reactions Part 1 Element Element Compound Youtube
(120).jpg)
Chemistry Mixtures Elements Compounds Quiz Proprofs Quiz

Elements Compounds And Mixtures Elements Compounds And Mixtures Compounds And Mixtures Compounds
How Are Elements And Compounds Different Quora
(29).jpg)
Chemistry Quiz Element Compound And Mixture Trivia Proprofs Quiz
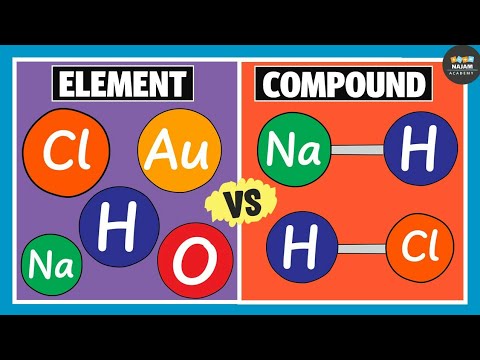
Difference Between Element And Compound Chemistry Youtube

Compounds Facts Science Trek Idaho Public Television

Percentage Composition Definition Examples Problems Chemistrygod

Elements And Compounds Texas Gateway

Elements Molecules And Compounds Practice Freebie Shape Tracing Worksheets Biology Worksheet Molecules

Elements And Compounds Introduction To Chemistry

Elements And Compounds Texas Gateway

Element Mixture Compound Activity Elements Compounds And Mixtures Heterogeneous Mixture Chemistry Classroom
Chemistry Elements Compounds And Mixtures