Covalent Compounds Are Formed Between What Type Of Elements
In general metallic elements tend to form ionic compounds and non-metallic elements tend to form covalent bonds. Ionic bonds are formed through the electrostatic forces between atoms that attract them towards each other due to opposite electrical charges.
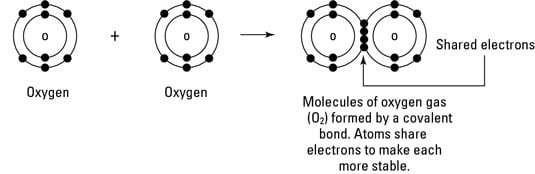
Environmental Science What Is Covalent Bonding Dummies
You understand how chemical bonds form and how electrons are transferred or shared to form ions and compounds.

Covalent compounds are formed between what type of elements. The most common type of bond formed by carbon is a covalent bond. For many molecules the sharing of electrons allows each atom to attain the equivalent of a full valence. Polar covalent is the intermediate type of bonding between the two extremes.
The strongest type of covalent bonds are sigma bonds which are formed by the direct overlap of orbitals from each of the two bonded atoms. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
Atoms with close. What are Ionic Compounds Ionic compounds are a result of ionic bonds. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic.
Ionic and covalent bonds are the two extremes of bonding. Each atom contributes an equal number of electrons towards the bond formation. Atoms with the same electronegativity like two oxygen atoms form nonpolar covalent bonds.
Predict the type of compound formed from elements based on their location within the periodic table Determine formulas for simple ionic compounds In ordinary chemical reactions the nucleus of each atom and thus the identity of the element remains unchanged. This is because carbon typically bonds with elements which have a similar electronegativity. Examples of covalent bonds formed by carbon include carbon-carbon carbon-hydrogen and carbon-oxygen bonds.
For example most carbon-based compounds are covalently bonded but can also be partially ionic. In most cases carbon shares electrons with other atoms usual valence of 4. Regardless of the atomic orbital type sigma bonds can occur as long as the orbitals directly overlap between the nuclei of the atoms.
If youre ever in doubt about the type of bonds formed between atoms look at their position on the periodic table. The covalent bond is a bond formed when two atoms share one or more electron pairs.

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

A Covalent Bond Is Formed When Two Atoms Share Electrons Covalent Bonds Usually Form Between Two Or More Nonmetals Co A Molecule Is A Neutral Ppt Download

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Bond An Overview Sciencedirect Topics

Covalent Bonding Biology Definition Role Expii

Covalent Compounds Manoa Hawaii Edu Exploringourfluidearth

Chemical Bonds Ionic Bonds Properties Types Of Covalent Bonds Science Online
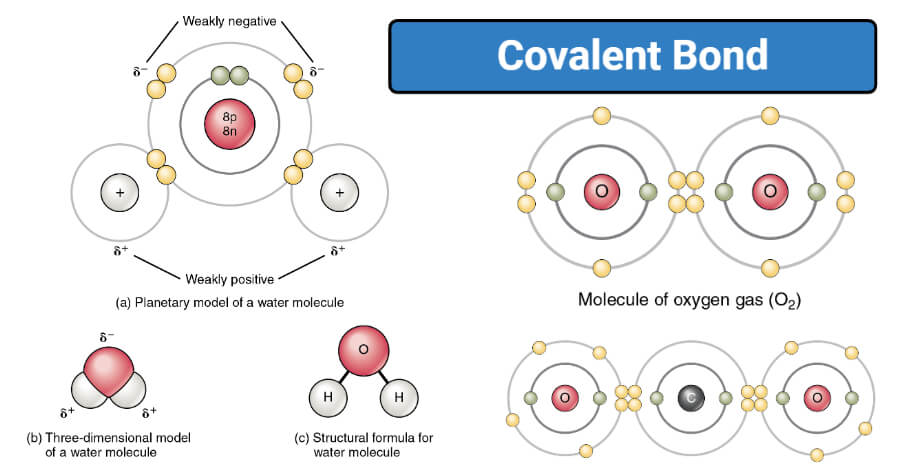
Covalent Bond Definition Properties Types Formation Examples
What Does A Covalent Bond Mean Quora

Covalent Bond Definition Types And Examples
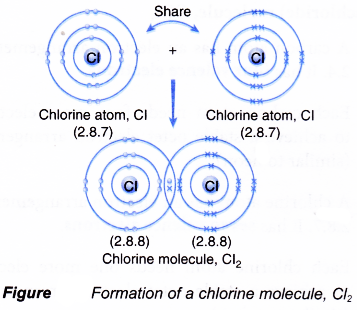
How Is Covalent Bond Is Formed A Plus Topper

Covalent Bond Biology Dictionary
Single And Multiple Covalent Bonds Article Khan Academy
How Is A Covalent Bond Formed In Water Quora

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Bond Examples Formation Properties What Is A Covalent Bond Video Lesson Transcript Study Com
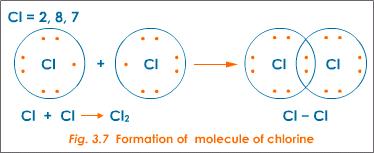
How Does The Formation Of Covalent Bonds Relate To The Octet Rule Socratic
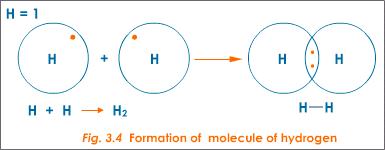
How Does The Formation Of Covalent Bonds Relate To The Octet Rule Socratic

Covalent Bond Definition Types And Examples
