Are Covalent Compounds Solid At Room Temperature
More significantly in solid-state chemistry we study the concept of a compound in a more deeper level. Inorganic compounds with simple ions typically have small ions and thus have high melting points so are solids at room temperature.

Question Video Comparing Molecular Solids And Covalent Networks Nagwa
Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons ionic compounds dissolved in water make an electrically conductive solution.

Are covalent compounds solid at room temperature. Usually an electron is more attracted to one. Furthermore whereas ionic compounds are good conductors of electricity when dissolved in water most covalent compounds are insoluble in water. In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
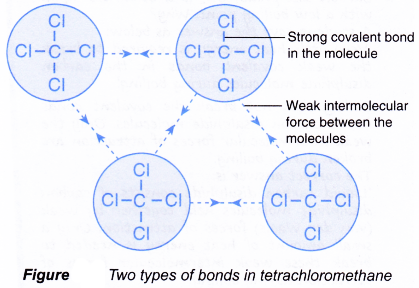
Substances that consist of covalent molecules are usually gases or liquids at room temperature. In a covalent bond the atoms are bound by shared electrons. Solid-state in Chemistry is the study of the structure properties and the synthesis of solid materials.
It is also sometimes called as materials chemistry. In fact many covalent compounds are liquids or gases at room temperature and in their solid states they are typically much softer than ionic solids. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
In the kinetic theory of gases the term molecule is often used. Some substances with larger ions however have a melting point below or near room temperature often defined as up to 100 C and are termed ionic liquids. Since they are electrically neutral they are.
Ionic compounds Ionic bonds are formed between a metal and non-metal for example sodium chloride. In a true covalent bond the electronegativity values are the same eg H 2 O 3 although in practice the electronegativity values just need to be closeIf the electron is shared equally between the atoms forming a covalent bond then the bond is said to be nonpolar. At room temperature and normal atmospheric pressure covalent compounds may exist as a solid a liquid or a gas whereas ionic compounds exist only as solids.
Molecules are distinguished from ions by their lack of electrical charge.

The Covalent Bond Boundless Chemistry

Ionic Vs Molecular Compounds Final Gcse Chemistry Chemistry Study Guide Physical Science

Menthol Is An Ingredient In Ricola French Words Wise Quotes School Scores

Covalent Compounds Covalent Bond Properties Examples With Videos
Properties Of Ionic And Covalent Compounds A Plus Topper

Ionic Bonding Worksheet Answer Key Photograph Very Well In 2021 Covalent Bonding Gcse Chemistry Teaching Chemistry

Synthesis Physical Vs Chemical Properties Changes Physical Vs Chemical Properties Teaching Chemistry Physics

Potassium Is A Metal So It Is A Solid At Room Temperature Potassium Metal Pocket Knife
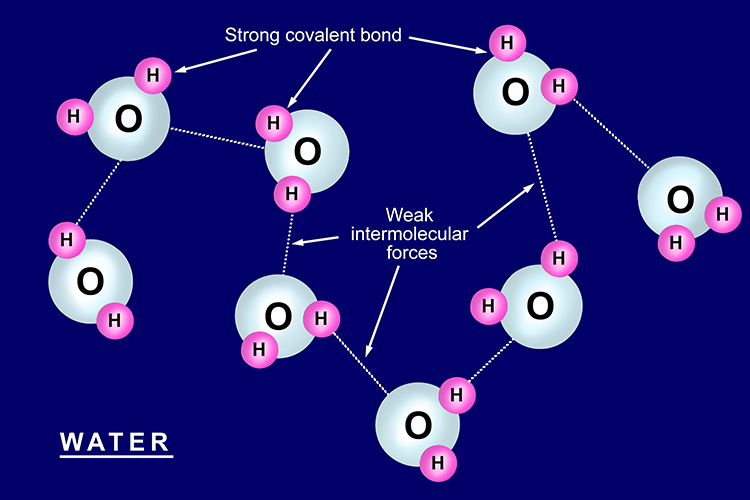
Properties Of Simple Covalent Bonded Substances

Why Do Some Solids Dissolve In Water But Others Do Not Why Are Some Substances Gases At Room Temperature But Othe Intermolecular Force Molecules Video Online

Afbeelding Overzicht En Vergelijking Van Eigenschappen Van Ionische En Covalente Verbindingen Teaching Chemistry Chemistry Classroom Chemistry Lessons

Study Study Study Study Study Side Up 2 00pm Sunday 5th April 2015 Study Notes Study Hard School Organization Notes

Pin By Jess Leblanc On School Year In 2021 Covalent Bonding Chemical Bond Metallic Bonding

Chemical Bonding Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science Science Lessons Chemical Bond Covalent Bonding
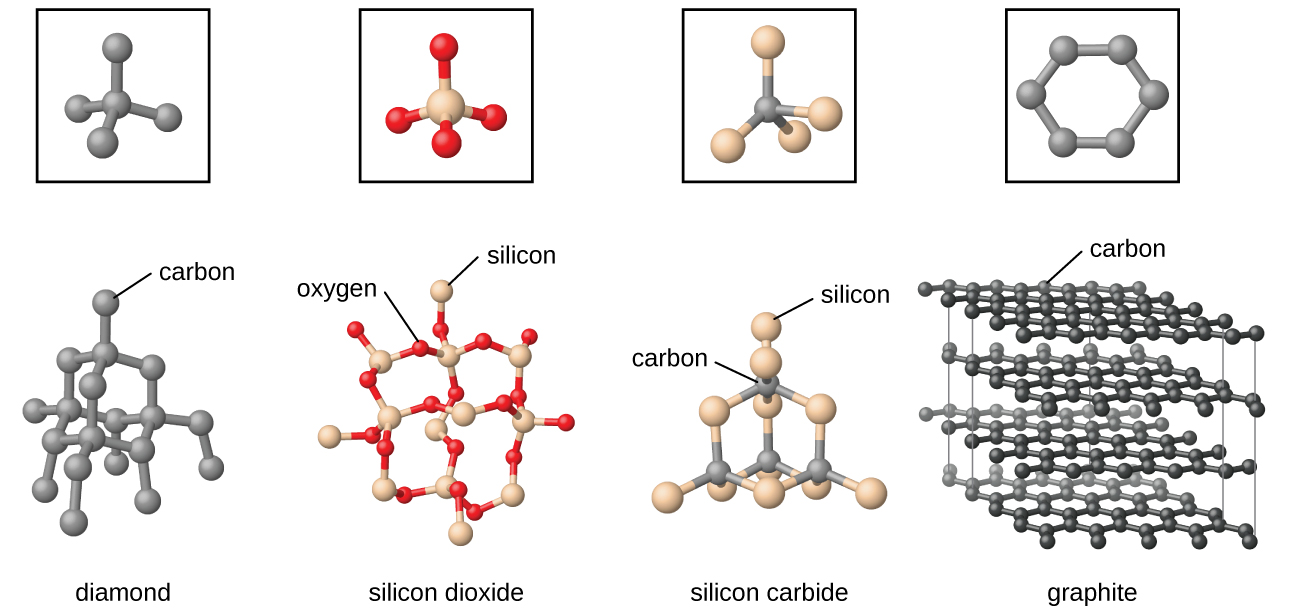
10 5 The Solid State Of Matter Chemistry

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts
Are All Ionic Compound Solid At Room Temperature Quora
The Covalent Bond Boundless Chemistry
Properties Of Covalent Compounds
