What Are The 3 Types Of Homogeneous Mixtures
Types of mixtures. We can observe only one phase of matter in a homogeneous mixture.

Homogeneous Mixture Definition Examples Tutors Com
Mixtures powerpoint 1.

What are the 3 types of homogeneous mixtures. There are two general types of mixtures. Some of the commonly used flash memory devices are. Examples include sand and sugar salt and gravel a basket of produce and a toy box filled with toys.
A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. In other words they are uniform throughout. The particles of the substances are mixed together there is no clumping of the particles eg air.
Pure substances and mixtures. It is the most commonly used device to store data because is more reliable and efficient as compare to other storage devices. Ask students to discuss their lists.
Mostly the difference between the two types of mixtures is a matter of scale. The packing provides a large surface area for vapor-liquid contact which increases the columns effectiveness. Some of the most recent and significant developments in homogeneous nickel catalysis are reviewed including nickel-mediated cross-coupling.
These are called aqueous solutions denoted aq in which water acts as a solvent. Homogeneous mixture examples include dissolving things like salt sugar or food coloring into water. When something dissolves it.
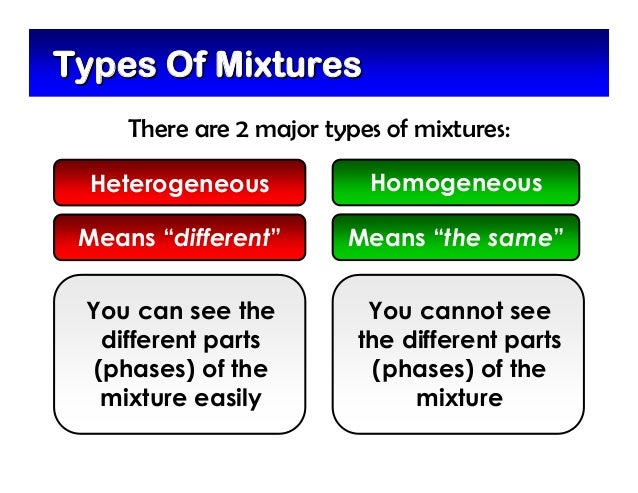
Based on the composition of mixtures they can be divided into two types. Another word for a homogeneous mixture is solutionThus a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. Particles of one substance the solute are mixed together with the particles of another substance the solvent.
Know more about mixtures and solutions with examples at BYJUS. Distinguishing between mixture types. Types of photocatalysis Homogeneous photocatalysis.
For instance a mixture of salt and water a mixture of sugar and water air lemonade soft drink water. Mixture means the thing which contains two or more different substances in any ratio such as the seawater granite and gasoline The mixtures can be classified according to their homogeneity into two types which are homogeneous solutions and heterogeneous colloids and suspensions. Types of Solutions - A solution is defined as a homogenous mixture comprising of two or more components of solute and solvent.
Column TypesConventional Distillation Packed Beds Although packed bed columns are used most often for absorption they are also used for the distillation of vapor-liquid mixtures. Alloys are homogeneous mixtures of metals. Mixtures can be classified as homogeneous or heterogeneous.
A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves as a single substance. If you look closely at sand from a beach you can see the different components including shells coral sand and organic matter. Heterogeneous and Homogeneous Definition What is a Homogeneous Mixture.
Matter can be classified into two broad categories. Mixtures having a uniform composition all through the substance are called Homogeneous Mixtures. On a coarse enough scale any mixture can be said to be homogeneous if the entire article is allowed to count as a sample of it.
A mixture is a combination of two or more substances where there is no chemical combination or reaction. It is a cheaper and portable storage device. These are the types of mixtures in which the components mixed are uniformly distributed throughout the mixture.
The most commonly used homogeneous photocatalysts include ozone and photo-Fenton systems Fe and Fe H 2 O 2. The two types of mixtures are distinguished by the size of the crystals that are present. Expect some to categorize the materials as elements mixtures and compounds At this point explain the different types of matter using the classroom board as needed.
Mixtures are physical combinations of two or more elements andor compounds. Homogeneous mixture Solution. Mixtures combine physically in no specific proportions.
Mixing together two solids without melting them together typically results in a heterogeneous mixture. Telling Homogeneous and Heterogeneous Mixtures Apart. The reactive species is the OH which is used for different purposes.
Solutions are homogenous mixtures. In homogeneous photocatalysis the reactants and the photocatalysts exist in the same phase. Then discuss all the materials on Table B again and separate them to classes of elements mixtures homogeneous and heterogeneous and.
Making a distinction between homogeneous and heterogeneous mixtures is a matter of the scale of sampling. Key points regarding such mixtures are.

Classifications Of Mixtures Heterogeneous Mixtures Composed Of Different Types Of Phases Of Substances Ex Fruit Salad Granite Homogeneous Mixtures The Ppt Download

Examples Of Homogeneous Mixtures Solid Liquid And Gas

Heterogeneous And Homogeneous Mixtures What S The Difference Homogeneous Mixture Examples Of Mixtures Heterogeneous Mixture
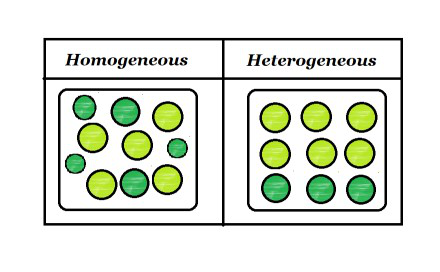
Homogeneous And Heterogeneous Mixtures Geeksforgeeks

Impure Substances Mixtures Ppt Video Online Download
Homogeneous Heterogeneous Mixture Definition Examples Selftution

3 4 Classifying Matter According To Its Composition Chemistry Libretexts

What Do You Need To Know About Heterogeneous And Homogeneous Mixtures
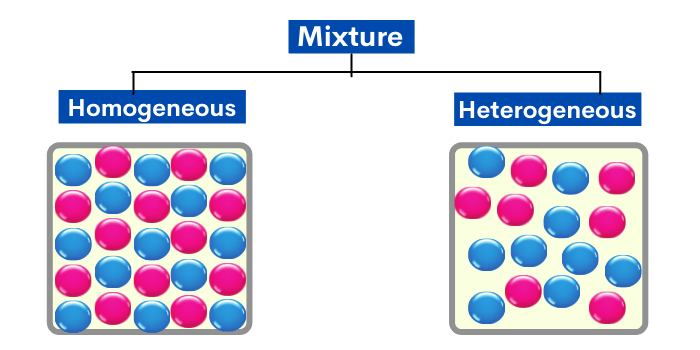
What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples
Homogeneous And Heterogeneous Mixture Nine Science
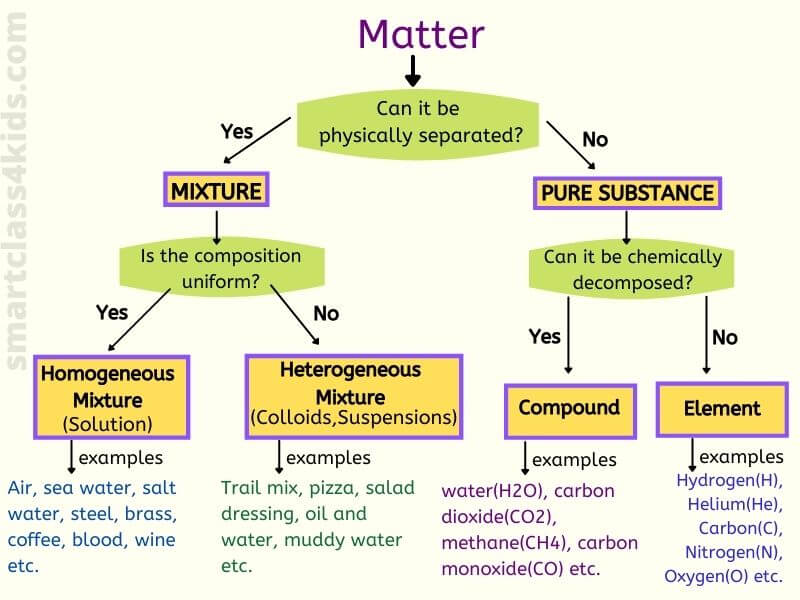
What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples

13 1 Types Of Mixtures Diagram Quizlet

Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool
What Kind Of Mixture Is Sand In Water Quora
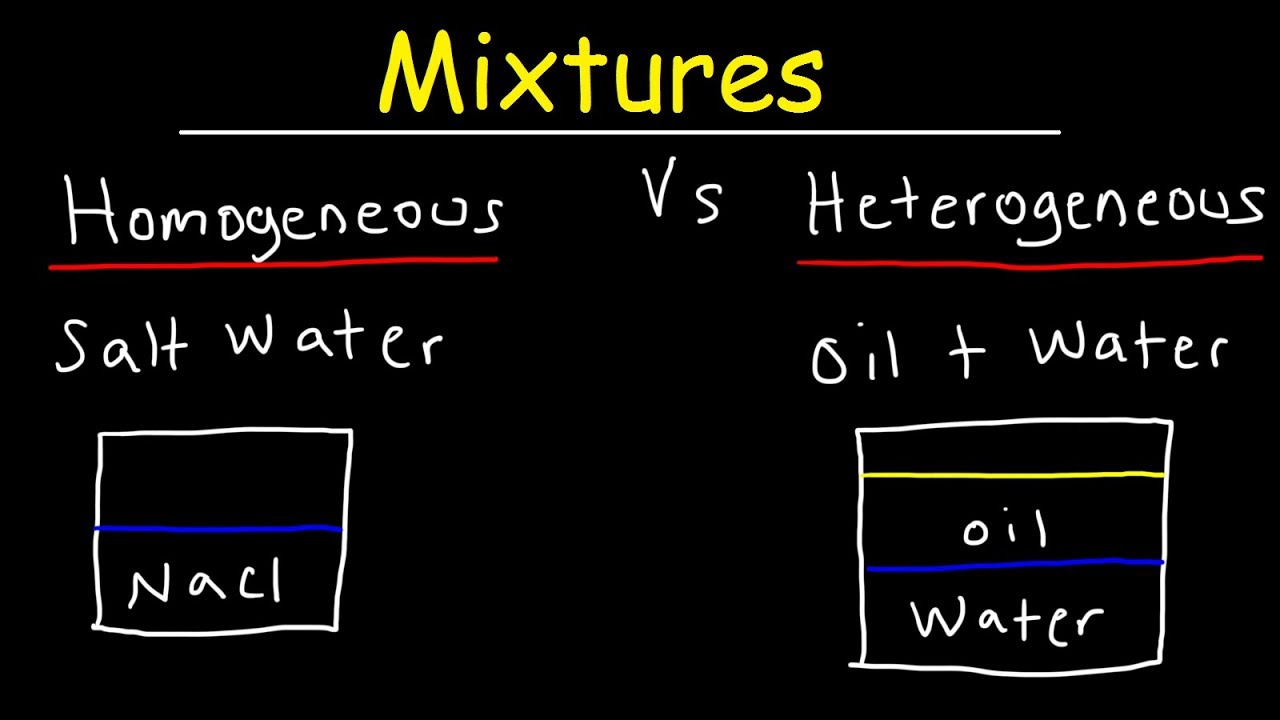
Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube

Solutions Types Of Mixtures Objectives 1 Distinguish Between Hetergeneous And Homogeneous Mixtures 2 List Different Solute Solvent Combinations 3 Compare Ppt Download
What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com

